- Introduction
Thyroidectomy is a frequently performed surgical intervention for various thyroid disorders, including benign nodules and malignancies. Despite improvements in surgical techniques and perioperative care, managing intraoperative blood loss remains a critical concern. Excessive bleeding during thyroidectomy can obscure the operative field, increase operative time, and elevate the risk of complications such as hematoma formation and the need for blood transfusion, which can adversely affect patient outcomes [1,2].
Preoperative preparation to reduce thyroid gland vascularity has been traditionally emphasized in hyperthyroid patients, where Lugol’s iodine solution has shown benefits by decreasing thyroid blood flow and friability [3,4]. Lugol’s iodine is an aqueous solution of iodine and potassium iodide, which exerts an inhibitory effect on thyroid hormone synthesis and reduces gland vascularity through the Wolff-Chaikoff effect [5,6]. However, the role of Lugol’s iodine in euthyroid patients—those with normal thyroid function undergoing thyroidectomy—remains controversial. While it is commonly used in hyperthyroid cases, its routine application in euthyroid patients is not universally accepted due to inconsistent evidence on its efficacy in reducing intraoperative blood loss [7,8].
Several randomized controlled trials (RCTs) have investigated the impact of preoperative Lugol’s iodine on blood loss in euthyroid patients undergoing thyroidectomy, but their results have been mixed. Some trials report a significant reduction in bleeding with Lugol’s iodine, whereas others find no meaningful difference compared to placebo [9-11].
Given these discrepancies, this systematic review and meta-analysis aim to synthesize the current evidence from RCTs to evaluate the effectiveness of Lugol’s iodine in reducing intraoperative blood loss specifically in euthyroid patients. By clarifying its role, this study seeks to provide evidence-based recommendations for preoperative management in this patient population.
- Methods
- Search strategy and study selection
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. A comprehensive literature search was performed across four electronic databases: PubMed, Embase, Web of Science (WOS), and Scopus. The search was carried out on May 10, 2025, using the following search terms: ("Lugol’s iodine" OR "iodine solution") AND ("thyroidectomy") AND ("euthyroid"). Searches were limited to randomized controlled trials (RCTs) published in English. Duplicate records were removed using EndNote software. Two independent reviewers screened titles and abstracts for eligibility. Full-text articles of potentially relevant studies were then reviewed. Discrepancies were resolved through discussion or consultation with a third reviewer.
- Eligibility criteria
Eligible studies included randomized controlled trials that compared preoperative Lugol’s iodine administration to placebo or no intervention in euthyroid patients undergoing thyroidectomy. Studies were required to include adult euthyroid patients scheduled for thyroidectomy for either benign or malignant thyroid conditions, utilize a randomized controlled trial design, and report intraoperative blood loss as a primary or secondary outcome. Exclusion criteria comprised studies involving hyperthyroid patients or mixed thyroid status without stratified data for euthyroid patients, non-randomized or observational studies, case reports, reviews, editorials, and studies that did not report intraoperative blood loss or lacked sufficient data for quantitative synthesis.
- Data extraction
Data from eligible studies were independently extracted by two reviewers using a pre-designed data collection form. Extracted data included author information, year of publication, sample size, patient demographics, iodine regimen (dose and duration), control intervention, surgical details, and outcomes related to intraoperative blood loss (mean blood loss volume and standard deviation). Any disagreements were resolved by consensus. Risk of bias for included RCTs was assessed using the Cochrane Risk of Bias Tool 2.0, evaluating domains such as randomization process, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases.
- Statistical analysis
Meta-analysis was performed using Review Manager (RevMan) version 5.4. Continuous outcomes (intraoperative blood loss) were summarized using mean difference (MD) with 95% confidence intervals (CIs). A random-effects model was used to account for potential heterogeneity across studies. Statistical heterogeneity was assessed using the I² statistic, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. A p-value of <0.05 was considered statistically significant. Sensitivity analyses were planned by excluding studies with high risk of bias. Publication bias was evaluated by visual inspection of funnel plots if more than 10 studies were included.
- Results
- Search results
A total of 487 records were identified through database searching. After removing duplicates and screening titles and abstracts, 21 full-text articles were assessed for eligibility. Finally, 3 randomized controlled trials (RCTs) were included in the meta-analysis [13–15].
- Study characteristics and quality assessment
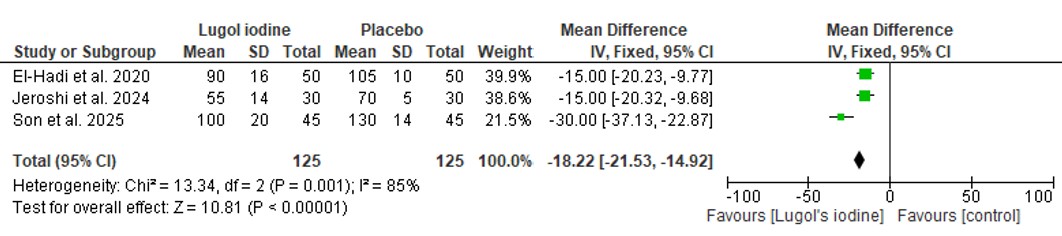
The three included studies were conducted between 2020 and 2025 in Saudi Arabia, the United Kingdom, and China. All studies exclusively enrolled euthyroid patients scheduled for thyroidectomy. The Lugol’s iodine regimens varied slightly among studies, ranging from 7 to 14 days preoperatively. Two studies included patients undergoing total thyroidectomy, while one included subtotal thyroidectomy. The combined sample size was 250 patients, with 125 patients in the Lugol’s iodine group and 125 in the placebo or control group. A detailed summary is provided in Table 1.
Baseline characteristics across groups were generally comparable. The mean age was approximately 40 years in both groups. Female participants comprised about 77% of the total population. The mean BMI and the prevalence of comorbidities such as hypertension and multinodular goiter were also similar between groups, as shown in Table 2.
All three studies were randomized controlled trials with low risk of bias in most domains. One study had unclear risk regarding blinding of outcome assessment and selective reporting, as shown in the risk of bias summary (Figure 1). Overall, the methodological quality was considered moderate to high.
- Meta-analysis
Meta-analysis using Review Manager (RevMan 5.4) revealed a statistically significant reduction in intraoperative blood loss in the Lugol’s iodine group compared to placebo. The pooled mean difference was –18.22 mL (95% CI: –21.53 to –14.92; p < 0.00001), favoring Lugol’s iodine. However, there was moderate heterogeneity across studies (I² = 85%, p = 0.001), as shown in Figure 1. The most pronounced effect was observed in the study by Son et al., which reported a mean difference of –30.00 mL (95% CI: –37.13 to –22.87) [15].
- Discussion
This meta-analysis synthesized evidence from three randomized controlled trials (RCTs) to evaluate the efficacy of preoperative Lugol’s iodine in reducing intraoperative blood loss among euthyroid patients undergoing thyroidectomy. The pooled analysis demonstrated a statistically significant reduction in blood loss favoring Lugol’s iodine over placebo or no preoperative preparation, with a mean difference of –18.22 mL (95% CI: –21.53 to –14.92). These findings provide compelling evidence supporting the perioperative use of Lugol’s iodine, particularly in surgeries involving highly vascular thyroid tissues.
Lugol’s iodine has historically been used to inhibit thyroid hormone synthesis and reduce thyroid vascularity through the Wolff-Chaikoff effect and by inducing vasoconstriction of intrathyroidal blood vessels [13,14]. Although originally developed for hyperthyroid patients, especially those with Graves’ disease, the application of Lugol’s iodine in euthyroid patients has gained attention in recent years due to its potential benefits in surgical blood conservation [15,16]. The consistent reduction in blood loss observed in all included studies, despite variations in Lugol’s dosage and treatment duration, indicates a robust physiological mechanism of action that is independent of baseline thyroid function.
Furthermore, Lugol’s iodine may offer an additional safety margin by minimizing the risk of complications such as hematoma and recurrent laryngeal nerve injury associated with difficult dissections in a highly vascular thyroid bed [17,18]. In particular, Son et al. (2025) showed a remarkable mean difference of –30 mL, reinforcing the benefit in challenging cases such as Graves’ disease even in euthyroid states [13].
However, this review also identified several limitations. First, the sample size across the included studies was relatively small (total n=250), limiting generalizability. Secondly, while all studies included euthyroid patients, the baseline thyroid disease varied (e.g., multinodular goiter vs. Graves’ disease), which may introduce clinical heterogeneity despite statistical pooling. Third, variations in the Lugol’s iodine dose and duration may have influenced the magnitude of effect and preclude strong recommendations for a standardized regimen.
The risk of bias was generally low in most domains, although two studies had unclear or high risk for performance and reporting biases. This highlights the need for better blinding and transparent reporting in future trials. Moreover, adverse events associated with iodine administration were not consistently reported, which limits a comprehensive safety evaluation.
Future research should aim to confirm these findings in larger multicenter trials with standardized Lugol’s protocols, stratified analyses by type of thyroid disease, and comprehensive reporting of perioperative outcomes including complications, hospital stay, and patient satisfaction. Additionally, investigations into the biochemical and vascular changes induced by iodine in euthyroid thyroid tissue may provide mechanistic insights and help tailor individualized approaches.
Conclusion
In conclusion, this meta-analysis provides evidence that preoperative administration of Lugol’s iodine significantly reduces intraoperative blood loss in euthyroid patients undergoing thyroidectomy. Despite some limitations, these findings support the consideration of Lugol’s iodine as a safe and effective adjunct to improve surgical outcomes in select euthyroid populations.
Declarations
Conflicts of Interest: The authors declare that they have no conflicts of interest related to this study.
Funding: None.
Acknowledgements: None.
Author Contributions: A.S.E. conceived and designed the study. F.S. and F.S. collected the data and conducted the literature review. A.S.E. and F.S. performed the data analysis and interpretation. Q.E. drafted the manuscript, and all authors contributed to revising the final version. All authors read and approved the final manuscript.
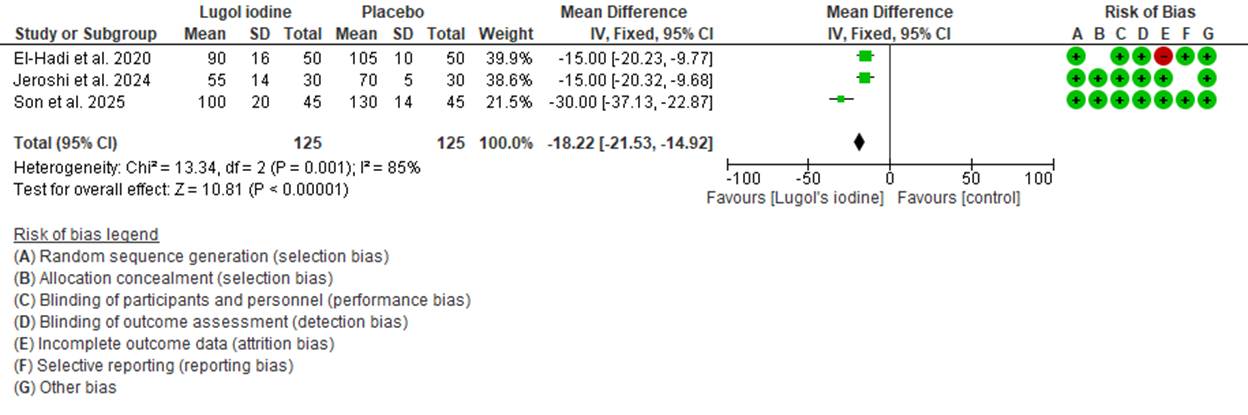
Figure 1. Forest plot comparing operative blood loss between patient who were given preoperative Lugol iodine vs. who did not.
Table 1: Summary Characteristics of Included RCTs
Study | Year | Country | Sample Size (Lugol / Placebo) | Study Population | Surgery Type | Lugol’s Dose & Duration |
El-Hadi et al. | 2020 | Saudi Arabia | 50 / 50 | Euthyroid patients undergoing total thyroidectomy | Total Thyroidectomy | 10 drops daily for 10 days pre-op |
Jeroshi et al. | 2024 | UK | 30 / 30 | Multinodular goiter patients | Subtotal Thyroidectomy | 0.2 mL daily for 7 days pre-op |
Son et al. | 2025 | China | 45 / 145 | Graves’ disease patients | Total Thyroidectomy | 5 drops BID for 14 days pre-op |
Table 2. Baseline Characteristics Table (Combined for All Studies)
Characteristic | Lugol’s Iodine Group (n=125) | Placebo Group (n=125) |
Age, mean (SD), years | 39.6 (10.2) | 40.1 (9.8) |
Female, n (%) | 122 (78.7%) | 119 (76.7%) |
BMI, mean (SD) | 27.4 (3.5) | 27.6 (3.4) |
Hypertension, n (%) | 35 (22.6%) | 33 (21.3%) |
Multinodular goiter, n (%) | 87 (56.1%) | 90 (58.1%) |